The Cell & Gene Therapy Connection
Cell therapy concentrates on manipulating an individuals cells to prevent, treat, cure or mitigate disease. The goal of cell therapy, overlapping with that of regenerative medicine, is to repair, replace or restore damaged tissues or organs. Gene therapy focuses on identifying genes in an individual which cause certain diseases & conditions and then manipulating or eradicating them. They are not the same thing, but are somewhat related in that both are used to cure and/or prevent certain diseases & conditions. Many medical researchers believe that these therapies have the potential to change the face of human disease and alleviate suffering.
What is cell therapy?
What are stem cells and why are they important?
What are embryonic stem cells?
What are adult stem cells?
Where do stem cells come from?
How is cell therapy being used today and what are the potential uses in the future?
What obstacles must be overcome before these potential uses will be realized?
What is gene therapy?
How does gene therapy work?
What is cell therapy?
The FDA defines cell therapy as, "The prevention, treatment, cure or mitigation of disease or injuries in humans by the administration of autologous, allogeneic or xenogeneic cells that have been manipulated or altered ex vivo."1 The goal of cell therapy, overlapping with that of regenerative medicine, is to repair, replace or restore damaged tissues or organs.
Cell therapy may take the form of a stem cell transplant such as a hematopoietic cell transplant that is used to restore the blood and immune system of patients with leukemia, lymphoma or other blood disorders.
Activation of the body's own immune system to fight cancer is referred to as adoptive immunotherapy. This type of cell therapy is most commonly used in the fight against cancer. One type of adoptive immunotherapy treatment artificially increases the number of T killer cells (a form of white blood cell) in the patient and involves collection of patient T cells, ex vivo expansion, then reinfusion to the patient. The result is an increase in the number of T cells and a stronger patient immune response to the cancer.
Regardless of the type of cellular therapy, production of the therapeutic product may require several complex techniques to alter or manipulate the cell. Cell engineering techniques may include:
- Propagation of cells
- Expansion of cells
- Selection of cells
- Pharmacological treatment of cells
- Alteration of biological characteristics of cells
What are stem cells and why are they important?
A stem cell is a cell (either adult or embryonic) that is capable of indefinite renewal through cell division and retention of its generic or unspecialized state while at the same time maintaining its potential to give rise to daughter cells of a more specialized type.
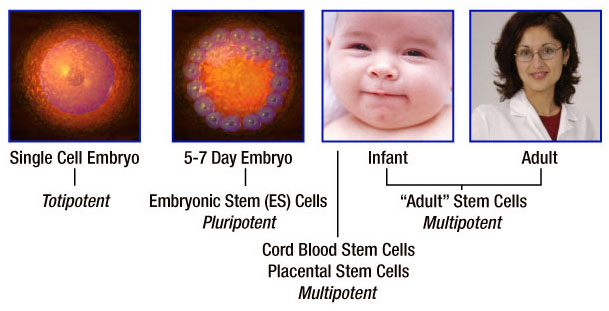
Classification of stem cells as totipotent, pluripotent and multipotent describes the breadth of the stem cells' ability to create specialized cell types. Stem cells represent a continuum or spectrum from embryonic stem cells to adult stem cells. The primary distinguishing factor is plasticity - the stem cell's capacity to differentiate into multiple specialized cell types.
Totipotent stem cells are known as the "master" cells of the body because they have the capacity to differentiate into the 216 specialized cell types that comprise the human body plus the placenta. A fertilized egg is an example of a totipotent cell.
Pluripotent stem cells are highly versatile cells and can give rise to any specialized cell type in the body except those needed to develop a fetus. Embryonic stem cells are pluripotent.
Multipotent stem cells can give rise to several specialized daughter cells but are limited to the particular tissue, organ or physiological system of origin. For example, hematopoietic stem cells can produce many types of blood cells in the circulatory system but cannot differentiate into a brain cell. Hematopoietic stem cells are an example of adult stem cells and are multipotent. Stem cells from umbilical cord blood are also multipotent based on evidence to date.
Take a look at totipotent, pluripotent and multipotent stem cells as they relate to the human development continuum:
Scientists expect enormous benefits from stem cell research and anticipate that it will revolutionize the practice of medicine. Stem cells may even lead to the creation of healthy tissues and organs for replacement of damaged and diseased body parts. Basic research to understand cell differentiation and human development may lead to a greater understanding of how birth defects occur. Stem cells may also be used during development of traditional pharmaceuticals. Stem cells stimulated to differentiate into specialized liver cells could be used for toxicology screening during the evaluation of drug candidates. Although the clinical benefits of cell-based therapies are already being seen, unlocking the full potential of stem cells will take decades of dedicated research. With each day, we move closer to realizing the promises of cell therapy and regenerative medicine.
What are embryonic stem cells?
Embryonic stem cells are derived from embryos, specifically the inner cell mass of a blastocyst, a hollow ball of cells that forms approximately five days after conception. Embryonic stems cells are the most primitive stem cells and as a result contain the most long-term promise for novel cell therapies and tissue regeneration.
Embryonic stem cells are pluripotent, meaning they have the ability to differentiate into any of the 200-plus cell types required by the body. Understanding and controlling embryonic stem cell differentiation and growth will require years of intensive research. Growing these cells in the laboratory is a time-consuming and painstaking process. Scientists must monitor embryonic stem cells closely and provide constant care to ensure continued growth and prevent uncontrolled or spontaneous differentiation.
Most embryonic stem cells used for research today have been donated from excess blastocysts created during in-vitro fertilization.
What are adult stem cells?
Adult (Somatic) stem cells are unspecialized cells that are found in different parts of the body and, depending on the source tissue, have different properties. Adult stem cells are capable of self-renewal and give rise to daughter cells that are specialized to form the cell types found in the original body part.
Adult stem cells are multipotent, meaning that they appear to be limited in the cell types that they can produce based on current evidence. However, recent scientific studies suggest that adult stem cells may have more plasticity than originally thought. Stem cell plasticity is the ability of a stem cell from one tissue to generate the specialized cell type(s) of another tissue. For example, bone marrow stromal cells are known to give rise to bone cells, cartilage cells, fat cells and other types of connective tissue (which is expected), but they may also differentiate into cardiac muscle cells and skeletal muscle cells (this was not initially thought possible).
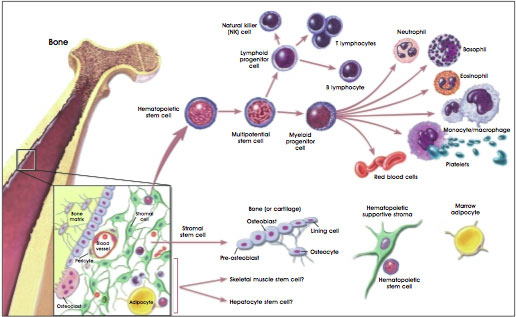
Hematopoietic stem cells that give rise to all blood and immune cells are today the most understood of the adult stem cells. Hematopoietic stem cells from bone marrow have been providing lifesaving cures for leukemia and other blood disorders for over 40 years. Hematopoietic stem cells are primarily found in the bone marrow but have also been found in the peripheral blood in very low numbers. Compared to adult stem cells from other tissues, hematopoietic stem cells are relatively easy to obtain.
Mesenchymal stem cells are also found in the bone marrow. Mesenchymal stem cells are a mixed population of cells that can form fat cells, bone, cartilage and ligaments, muscle cells, skin cells and nerve cells.
Hematopoietic and stromal stem cell differentiation:
Umbilical cord blood from newborns is a rich source of hematopoietic stem cells. Research has found that these stem cells are less mature than other adult stem cells, meaning that they are able to proliferate longer in culture and may contribute to a broader range of tissues. Research is ongoing to determine whether umbilical cord stem cells are pluripotent or multipotent and the extent of their plasticity.
Cord blood, which traditionally has been discarded, has emerged as an alternative source of hematopoietic stem cells for the treatment of leukemia, lymphoma and other lethal blood disorders. It has also been used as a life-saving treatment for children with infantile Krabbe's disease, a lysosomal storage disease that produces progressive neurological deterioration and death in early childhood.
Regardless of the adult stem cells' source - bone marrow, umbilical cord blood or other tissues - these cells are present in minute quantities. This makes identification, isolation and purification challenging. Scientists are currently trying to determine how many kinds of adult stem cells exist and where they are located in the body.
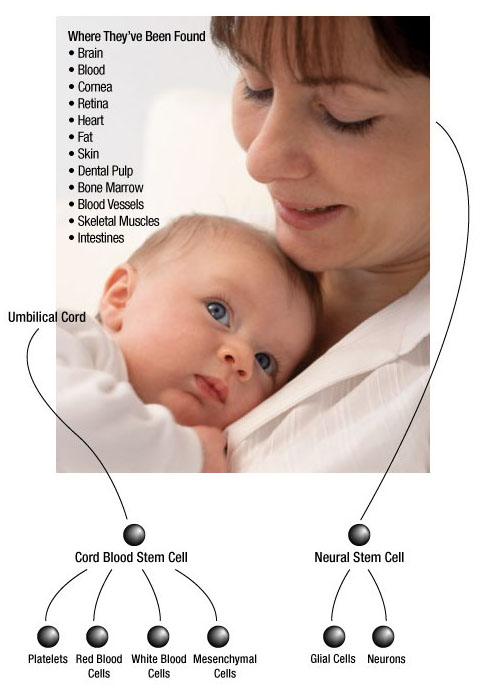
Where do stem cells come from?
Multipotent stem cells for transplant from bone marrow were used experimentally from the 1950's and 1960's with the work of Thomas and Storb and others, leading to stem cell transplant in the 1970's for hematologic malignancies. This work coined the term "stem cell." The first human pluripotent cells were isolated in 1998 by Dr. James Thompson at the University of Wisconsin. These cells were isolated from excess embryos obtained from in-vitro fertilization clinics. Subsequently, scientists have isolated stem cells from a variety of adult tissues, but these are multipotent, not pluripotent.
Adult stem cells have been found in the bone marrow, peripheral blood and umbilical cord blood. More recently, scientists have found stem cells in fat, skeletal muscle, skin, blood vessels, retina, liver, pancreas and the brain. Adult stem cells are extremely rare and difficult to identify, isolate and purify.
How is cell therapy being used today and what are the potential uses in the future?
Bone marrow transplants have been used for the past 40 years to regenerate the blood and immune systems of patients with leukemia, lymphoma, severe aplastic anemia or inherited metabolic diseases. Unfortunately, the major limitation with allogenic bone marrow transplants is the availability of matched donors.
Stem cells from Umbilical Cord Blood (UCB) have emerged as an alternative to bone marrow transplants, providing an easily obtainable and readily available source of treatment. UCB transplants may result in a lower incidence of transplant complications, specifically graft-versus-host disease, common in patients receiving a transplant from an unrelated donor. UCB transplants also have less stringent requirements for donor matching compared to bone marrow transplants, increasing the likelihood that an appropriate donor can be found for patients. Until recently, UCB transplants were limited to pediatric patients due to the low cell stem cell dose. But in 2004, researchers demonstrated that combining stem cells from two UCB units could increase the cell dose to extend this lifesaving hematopoietic treatment to adult patients.
In addition to regenerating the blood and immune systems, scientists anticipate that stem cells will be used to replace damaged or diseased tissues and organs. Clinical trials are ongoing to repair scarred or dying heart muscle after a heart attack or during congestive heart failure. On-going research in diabetes is focused on understanding how stem cells might be trained to become the type of pancreatic islet cells that secrete needed insulin. Repair of debilitating spinal cord injuries is also a goal of researchers through the regeneration of neurons, myelin and nerve cells.
As basic research continues, researchers hope to learn how cells replicate and give rise to specialized daughter cells, which may provide insight into inborn cell errors that cause birth defects. Understanding cell signaling pathways can also provide clues to how stem cells are able to hone in on the site of injury to initiate repair of damaged or diseased tissues.
Harnessing stem cells for use as drug delivery systems is another goal of researchers. Stem cells may be able to bring chemotherapeutic agents directly to the targeted cancerous cells. Stem cells may also be used to generate liver cells or other tissues that can be used in screening new drug candidates for safety in pharmaceutical drug development. Using human cells and/or tissues may provide a better model for toxicology testing than the traditional animal models in use today.
Cancer vaccines, a type of adoptive immunotherapy, are in clinical trials for prostrate, breast, ovarian and colorectal cancers. Combining tumor cells from the patient with dendritic cells can lead to a vaccine that will seek out and destroy the cancerous cells. Other adoptive immunotherapies can artificially increase the number of T killer cells (a form of white blood cell) in a cancer patient. This involves collection of patient T cells, ex vivo expansion, then reinfusion to the patient. The result is an increase in the number of T cells and a stronger patient immune response to the cancer.
What obstacles must be overcome before these potential uses will be realized?
Before cell therapies move from basic research laboratories and into widespread use in the clinic, several technical obstacles must be overcome. Scientists must be able to:
- Understand and control the mechanism of turning undifferentiated cells into specialized cells. This involves identifying the complex signals needed to turn the genes on and off that initiate and govern the differentiation of cells.
- Identify, isolate and purify different adult stem cell types. Purified and/or expanded stem cells will be required for safe, efficacious treatments.
- Control the differentiation of stem cells to target cell types needed to treat disease such that sufficient quantities of the correct stem cell or differentiated cell can be generated for treatment.
- Learn to make stem cell transplants patient-compatible to avoid rejection by the immune system.
- Demonstrate clinical improvement and normal cell development and function once stem cells have been transplanted into the patient's body. Stem cells must become integrated with the patient's own tissues and learn to function as one of the patient's natural body cells.
What is gene therapy?
The FDA defines cell therapy as, "The prevention, treatment, cure or mitigation of disease or injuries in humans by the administration of autologous, allogeneic or xenogeneic cells that have been manipulated or altered ex vivo (in a controlled environment outside of the body)." The goal of cell therapy, overlapping with that of regenerative medicine, is to repair, replace or restore damaged tissues or organs.
Other types of gene therapy include delivery of RNA or DNA sequences (oligonucleotide therapy) that can be used either to depress function of an unwanted gene, such as one responsible for a mutant protein which acts in a negative way to reduce normal protein function (usually inherited in an autosomal dominant manner), to try to correct a defective gene through stimulation of DNA repair within cells, or to suppress an oncogene which acts as a driver in a cancer cell.
In other strategies for diseases and cancer, the gene/RNA/DNA delivered is a novel agent intended to change the metabolic state of the cells, for example to make cancer cells more susceptible to drug treatment, to keep dying cells alive by delivery of growth factors, to suppress or activate formation of new blood vessels or to increase production of a critical metabolite, such as a neurotransmitter critical to brain function. Vectors and cells can also be used to promote an immune response to tumor cells and pathogens by expressing theses antigens in immune responsive cells in combination with factors which enhance the immune response.
How does gene therapy work?
Scientists focus on identifying genes that affect the progression of diseases. Depending on the disease, the identified gene may be mutated so it doesn't work. The mutation may shorten the protein, lengthen the protein, or cause it to fold into an odd shape. The mutation may also change how much protein is made (change its expression level). After identification of the relevant gene(s), scientists and clinicians choose the best current strategy to return cells to a normal state, or in the case of cancer cells, to eliminate them.
Thus, one aim of gene therapy can be to provide a correct copy of its protein in sufficient quantity so that the patient's disease improves or disappears. Five main strategies are used in gene therapy for different diseases and cancer: gene addition, gene correction, gene silencing, reprogramming, and cell elimination. In some common diseases, such as Parkinson's disease and Alzheimer's disease, different genes and non-genetic causes can underlie the condition. In these cases, gene/cell therapy can be directed at the symptoms, rather than the cause, such as providing growth factors or neutralizing toxic proteins.
Gene addition involves inserting a new copy of the relevant gene into the nucleus of appropriate cells. The new gene has its own control signals including start and stop signals. The new gene with its control signals is usually packaged into either viral vectors or non-viral vectors. The gene-carrying vector may be administered into the affected tissue directly, into a surrogate tissue, or into the blood stream or intraperitoneal cavity. Alternatively, the gene-carrying vector can be used in tissue culture to alter some of the patients' cells which are then re-administered into the patient.
Gene therapy agents based on gene addition are being developed to treat many diseases, including adenosine deaminase severe combined immunodeficiency (ADA- SCID), alpha-antitrypsin deficiency, Batten's disease, congenital blindness, cystic fibrosis, Gaucher's disease, hemophilia, HIV infections, Leber's congenital amaurosis, lysosomal storage diseases, muscular dystrophy, type I diabetes, X linked chronic granulomatous disease, and many others.
Gene correction involves delivering a corrected portion of the gene with or without supplemental recombinant machinery that efficiently recombines with the defective gene in the chromosome and corrects the mutation in the genome of targeted cells. This can also be carried out by providing DNA/RNA sequences that allow the mutated portion of the messenger RNA to be spliced out and replaced with a corrected sequences or, when available in the genome, increasing expression of a normal counterpart of the defective gene which can replace its function.
Gene silencing reduces the expression of the target gene, typical by disrupting translation of the messenger RNA encoded in it through interfering RNA molecules. In some cases, the diseased tissue produces too much protein from a specific gene and this overly abundant production is associated with symptoms of the disease. The interfering RNA binds to the normal RNA of the gene and blocks its translation into protein. These interfering RNAs can be synthetic (oligonucleotide therapy) or encoded in novel genes that make sequences that are the inverse of the normal sequence (antisense) and can thus hybridize to the message and prevent its translation. Cells normally make microRNAs which perform this function as a normal form of regulation of gene expression, sometimes resulting in degradation of the targeted message. By changing levels of specific microRNAs in cells, one can also achieve downregulation of gene expression. Thus, interfering RNAs reduce protein production of the corresponding gene.
For example, too much tumor necrosis factor (TNF) alpha is often expressed in the afflicted joints of rheumatoid arthritis patients. Since the protein is needed in small amounts in the rest of the body, gene silencing aims to reduce TNF alpha only in the afflicted tissue. Another example would be oncoproteins, such as c-myc or EGFR that are upregulated or amplified in some cancers. Lowering expression of these oncoproteins in cancer cells can inhibit tumor growth.
Reprogramming involves the addition of one or more genes into cells of the same tissue which causes the altered cells to have a new set of desired characteristics. For example, type I diabetes occurs because many of the islet cells of the pancreas are damaged. But the exocrine cells of the pancreas are not damaged. Several groups are deciphering which genes to add to some of the exocrine cells of the pancreas to change them into islet cells, so these modified exocrine cells make insulin and help heal type I diabetic patients.
This is also the strategy in the use of induced pluripotent stem cells (iPS) where skin cells or bone marrow cells are removed from the patient and reprogrammed by transitory expression of transcription factors which turn on developmentally programmed genes, thereby steering the cells to become the specific cell types needed for cell replacement in the affected tissue.
Cell elimination strategies are typically used for cancer (malignant tumors) but can also be used for overgrowth of certain cell types (benign tumors). Typical strategies involve suicide genes, anti-angiogenesis agents, oncolytic viruses, toxic proteins or mounting an immune response to the unwanted cells. Suicide gene therapy involves expression of a new gene, for example an enzyme that can convert a pro-drug (non-harmful drug precursor) into an active chemotherapeutic drug. Expression of this suicide gene in the target cancer cells can only cause their death upon administration of a prodrug, and since the drug is generated within the tumor, its concentration is higher there and is lower in normal tissues, thus reducing toxicity to the rest of the body. Since tumors depend on new blood vessels to supply their ever increasing volume, both oligonucleotides and genes aimed at suppressing angiogenesis have been developed.
In another approach, a number of different types of viruses have been harnessed through mutations such that they can selectively grow in and kill tumor cells (oncolysis), releasing new virus on site, while sparing normal cells. In some cases toxic proteins, such as those that produce apoptosis (death) of cells are delivered to tumor cells, typically under a promoter that limits expression to the tumor cells.Other approaches involve vaccination against tumor antigens using genetically modified cells which express the tumor antigens, activation of immune cells or facilitation of the ability of immune cells to home to tumors.
Cancer therapy has been limited to some extent by the difficulty in efficient delivery of the therapeutic genes or oligonucleotides to sufficient numbers of tumor cells, which can be distributed throughout tissues and within the body. To compensate for this insufficient delivery, killing mechanisms are sought which have a "bystander effect" such that the genetically modified cells release factors that can kill non-modified tumor cells in their vicinity. Recent studies have found that certain cell types, such as neuroprecursor cells and mesenchymal cells, are naturally attracted to tumor cells, in part due to factors released by the tumor cells. These delivery cells can then be armed with latent oncolytic viruses or therapeutic genes which they can carry over substantial distances to the tumor cells.